From this figure, we might conclude that Venus would be "just right" and Earth would be too cold. But this ignores the extremely important effect of an atmosphere in warming the surface. This effect is called the greenhouse effect.
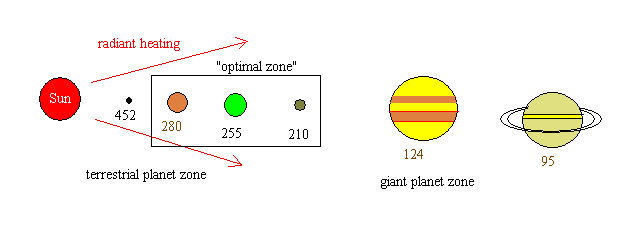
Here, once again, is a sketch
showing the temperatures that would be reached by airless
bodies at various points in the solar system. The
temperatures are maintained by solar heating. There is a
zone near 1 AU from the Sun where such temperatures are close to
300 K, the so-called "optimal zone" or "comfort zone":

From this figure, we might conclude
that Venus would be "just right" and Earth would be too
cold. But this ignores the extremely important effect of
an atmosphere in warming the surface. This effect is
called the greenhouse effect.
If the Earth were off in interstellar space and all of the volatile compounds (such as N2, O2, Ar, and H2O) in its atmosphere condensed to form a solid sheet of ice on its surface, the sheet would only be something like 10 meters thick! That is not very much protection against bombardment from space!
The scale height of an atmosphere is the way that planetary scientists represent its actual thickness. Here is a diagram that explains the concept of a scale height (usually denoted by H):
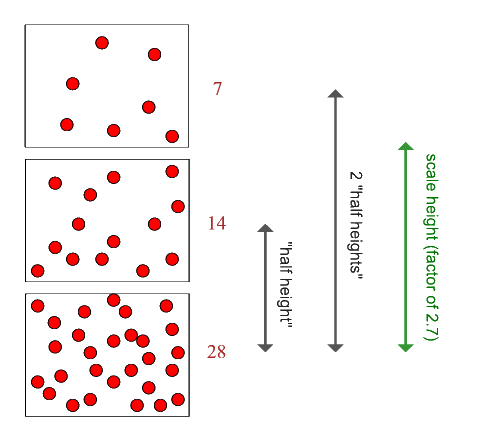
Near the surface, the Earth's atmosphere has a scale height of about 8 km. If you want to think of the effective thickness of the Earth's atmosphere, remember a number of about 10 km.
Greenhouse
About 45% of incoming solar radiation is absorbed by the ground. What happens to this radiation? The Earth has to radiate it back to space at infrared wavelengths, as heat. We can see this emitted energy when we look at GOES infrared satellite images. These infrared images are available at all times, even when the surface is in darkness. Notice that the GOES infrared images are shaded so that regions that are emitting a lot of infrared are dark, and regions that are not emitting much are bright (this is sort of opposite to the visible images). Now at wavelengths that are sensitive to water vapor, we see bright shading where there is a lot of water vapor. In fact the surface of the Earth is still emitting below this vapor, but it can't get into space easily. So the heat builds up.
As a result, water vapor (H2O)
is called a greenhouse gas. It causes infrared
energy (heat) to be trapped near the surface of the Earth, and
is partly responsible for the troposphere. It also allows
liquid water to exist at the Earth's surface, so it is essential
for life.
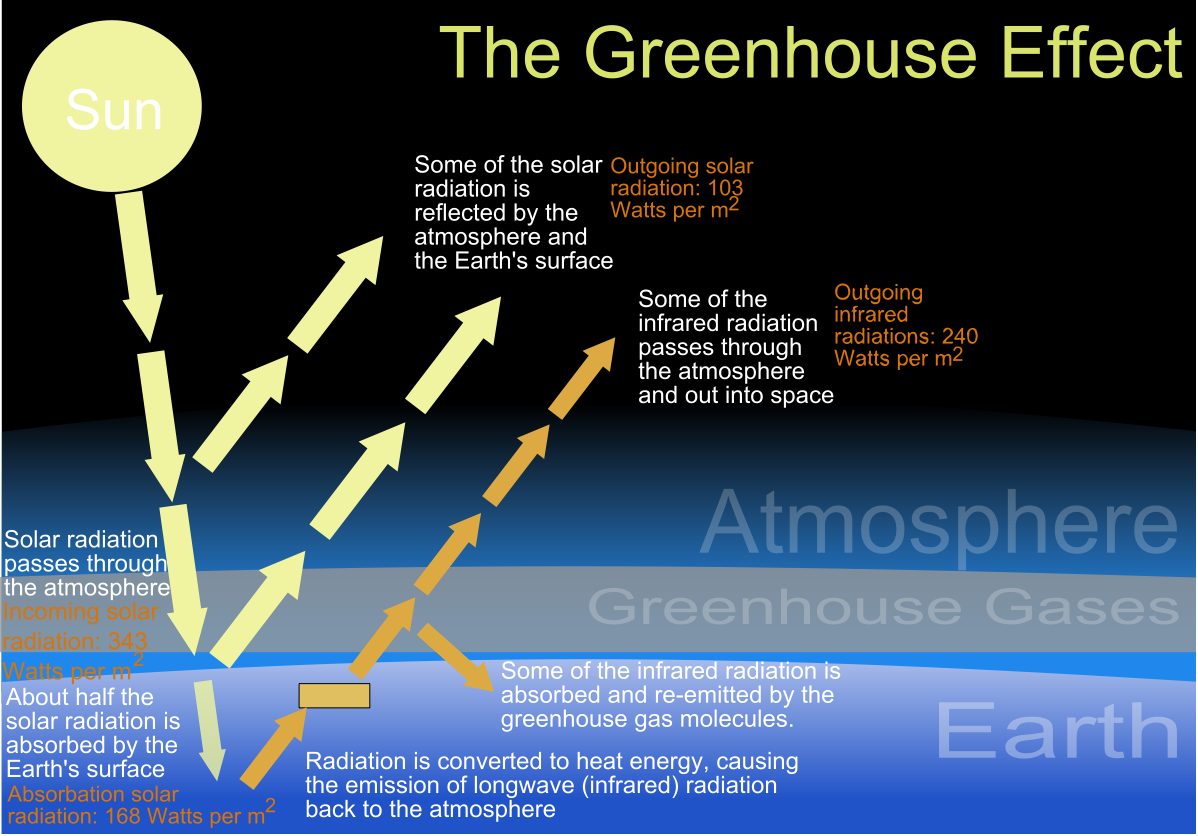
image from the
Wikimedia Commons
Although water vapor is responsible for most of the Earth's atmospheric greenhouse effect, some of the effect comes from other greenhouse molecules, in particular CO2 (carbon dioxide) and CH4 (methane).
Atmospheric escape
The tendency of an atmosphere to escape into space can be expressed by this ratio:
H / a,
where a is the planet's radius (6374 km for Earth). If this ratio is small, the atmosphere is held tightly by the planet. If it is not so small (compared with 1), then the atmosphere tends to escape into space.
Now H depends on three
quantities that characterize the atmosphere:
Recall:
the force F between two
bodies, one of mass m and the other of mass M,
is given by
F = -GMm/ r2,
where r is the distance between the two bodies and G is a constant number called the Universal Gravitational Constant, or just gravitational constant. The minus sign is a convention that means the force is attractive -- it tries to pull the two bodies together.
The surface gravity is given by
g = -GM/ r2,
so
F = gm,
where F is the weight of the mass m.
Now
So what all this tells us, is:
Composition
of Earth's atmosphere
There is an unusual amount (21%) of
O2 in the Earth's atmosphere:
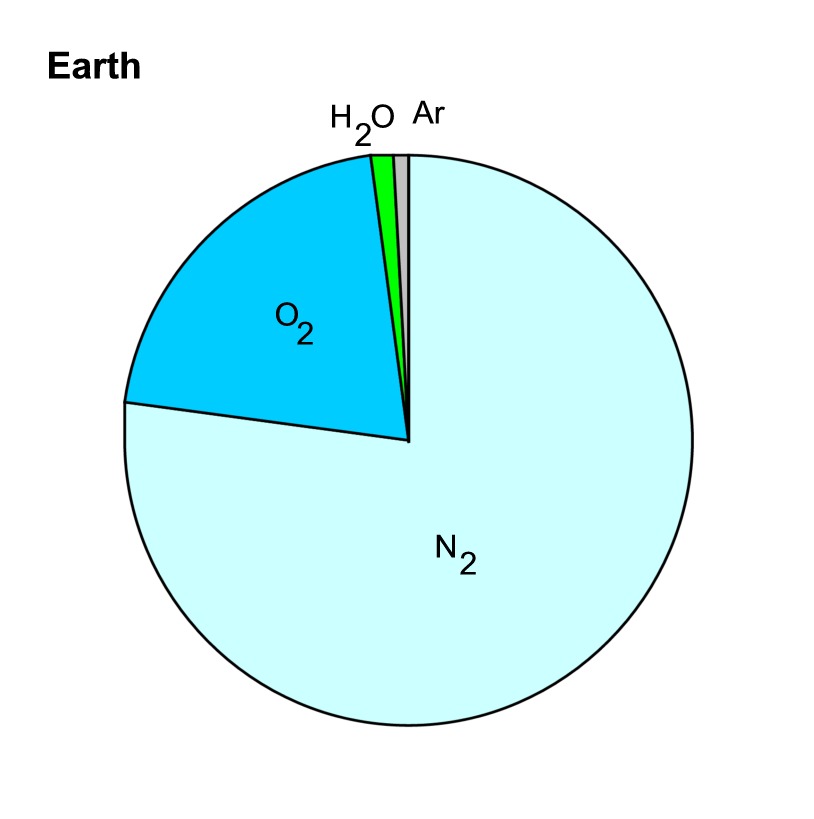
Together, O2 and N2
amount about 99% of the air we breath. The remaining 1%
has two big components. Argon (in the form of Ar40,
mostly from decay of K40) is a little less
than 1%, but is constant. Water vapor (H2O gas)
is a little less than 1% on
average, but is highly
variable, as we know from living here in Tucson.
There are other important components as well, even though they
are very minor gases (much less than 1% by mixing fraction),
such as carbon dioxide (CO2) and methane (CH4).
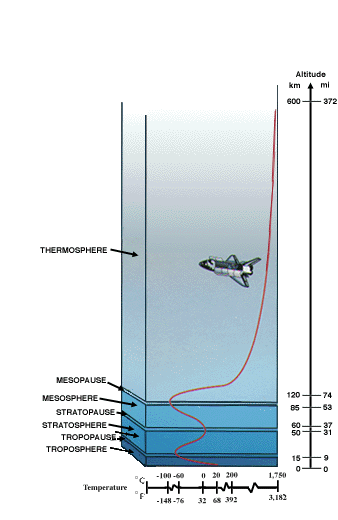
Structure of the Earth's atmosphere
The Role of Water
Now let's talk about the important role of water in the evolution of terrestrial planet atmospheres. As we said, water is, on average, about 1% of the Earth's atmosphere. The surface of the Earth is in a special part of the water phase diagram -- it is near the triple point of water:
The critical point of water
is the point above which there is no distinction between liquid
and vapor -- it's just one big hot gas-fluid phase. Most
substances have critical points at various temperatures and
pressures. The location of present-day conditions on Mars
is shown. Notice that there is no possibility for liquid
water on Mars. The water just sublimes directly
from the ice phase to the vapor phase. All water clouds on
Mars are composed of ice crystals.